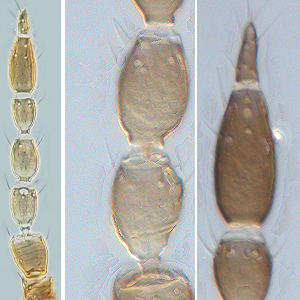
Microcephalothrips abdominalis (Crawford DL, 1910)
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 7-segmented antenna, segments III and IV with forked sense cone, terminal segments VI and VII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Fore wing and fore wing basal region
Fig. 6: Tergites V and VI
Fig. 7: Sternites VI and VII
Fig. 8: Tergites VIII and IX
Fig. 9: Posteromarginal comb of tergite VIII and tergites IX-XI
Introduction and recognition
Microcephalothrips abdominalis breeds in the flowers of various Compositae like marigold (Tagetes sp.), Pyrethrum (Chrysanthemum sp.), and tropical whiteweed. Female macropterous or micropterous; body color brown; fore tibiae, tarsi and antennal segment III and sometimes IV paler; fore wings brown with a paler area sub-basally. Antennae 7-segmented; segments III & IV with small forked sense cone (Fig. 1). Head wider than long; only 2 pairs of ocellar setae present; setae I absent, setae III subequal in length to pair II and arise anterolateral to ocellar triangle; postocular setae small (Fig. 2). Pronotum little wider at posterior than anterior margin (Fig. 3); with 2 pairs of short posteroangular setae; posterior margin with 5-6 pairs of setae (Fig. 3). Mesofurca with spinula. Metanotum with transverse lines at anterior, and linear sculpture forming lens-like shape at posterior; median setae arise behind anterior margin; campaniform sensilla present (Fig. 4). Mid and hind tarsi 2-segmented. Fore wing first vein with 3 setae on distal half; second vein with a complete row of about 7 setae; clavus with 5 marginal setae (Fig. 5). Tergites with sculpture lines medially on anterior half but not on posterior half; campaniform sensilla posterior to median setae (Fig. 6); paired ctenidia present on tergites V-VIII, on VIII posteromedial to spiracle; tergites posterior margin with craspedum of independent triangular lobes or teeth; complete posteromarginal comb on tergite VIII with slender microtrichia arising from broadly triangular bases (Fig. 8 and 9). Sternites without craspeda; with double row of 14-25 discal setae; median pair of marginal setae on sternite VII arise in front of margin (Fig. 7).
Male similar to female but smaller, paler and sometimes micropterous; tergite VIII posterior margin with craspedum of triangular lobes similar to preceding segments; sternites with craspeda of triangular lobes, sternites III-VII with small circular glandular area.
Second instar larva white, antennal segments faintly grey; tergites with transverse rows of small linear plaques, major setae with expanded capitate apices; tergite VIII spiracles surrounded by about 8 small cells; campaniform sensilla on IX anterior to median pair of setae, posterior margin with about 10 small tubercles.
Taxonomic identity
Species
Microcephalothrips abdominalis (Crawford DL, 1910)
Taxonomic history
Microcephalothrips yanglinensis Feng, Zhang & Sha, 2002
Microcephalothrips jigonshanensis Feng, 1998
Microcephalothrips chinensis Feng, 1998
Aureothrips marigoldae Raizada, 1966
Microcephalothrips brevipalpis armatus Ananthakrishnan, 1956
Thrips oklahomae Watson, 1931
Paraphysopus burnsi Girault, 1927
Stylothrips brevipalpis Karny, 1927
Thrips (Ctenothripiella) gillettei Moulton, 1926
Thrips microcephalus Priesner, 1923
Thrips crenatus Watson, 1922
Thrips femoralis Jones, 1912
Thrips abdominalis Crawford DL, 1910&xnbsp;
Common name
Composite thrips
Sunflower thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Microcephalothrips Bagnall, 1926
Genus description
The genus Microcephalothrips Bagnall, 1926
Only 1 species are listed in this genus, Microcephalothrips abdominalis, a small brown species is found throughout the tropics and subtropics. It is closely related to the genus Thrips, because it lacks ocellar setae I and has well developed tergal ctenidia, posteromedial to the spiracles on tergite VIII (Mound & Marullo 1996). Microcephalothrips abdominalis has a distinctive craspedum of tooth-like lobes on the posterior margin of the tergites, and the head is unusually small in comparison to the pronotum. Furthermore, the species has 7-segmented antennae with small forked sense cone on segments III and IV, a pronotum with 5-6 pairs of posteromarginal setae (Mound & Kibby 1998), and a complete posteromarginal comb with slender microtrichia arising from broadly triangular bases on tergite VIII.
Species description
Typical key character states of
Microcephalothrips abdominalis
Coloration and body sculpture
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Body color: mainly brown to dark brown
Antennae
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Number of antennal segments: 7
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: symmetric
Antennal segment III shape: symmetric
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Forked sense cone on antennal segment IV: scarcely extending beyond base of segment V
Antennal segment IV and V: without a hyaline ring near the base
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Head
Distance between bases of ocellar setae III: greater than width of first ocellus
Head: not prolonged in front of compound eyes
Ocellar setae I: absent
Length of ocellar setae II: shorter than setae III or S2 = S3
Ocellar setae III: arising on anterior margin of, or in front of ocellar triangle
Ocelli: present
Length of postocular setae: not alternating short and long setae
Number of ocellar setae: 2
Prothorax
Number of pairs of long anteroangular setae: 0
Number of pairs of long posteroangular setae: 2
Number of pairs of elongate pronotal setae: 2
Number of pairs of posteromarginal minor setae: 5-6
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Mesosternal furca: with median spinula
Metathorax
Metanotal campaniform sensilla: present
Metanotal median setae: S1 behind anterior margin
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: without spinula
Sculpture of metanotum median area: transverse at anterior, but longitudinal and parallel on posterior half
Shape of metathoracic furca: transverse, V-shaped
Wings
Fore and hind wings: present, more than half as long as abdomen (macropterous) or not longer than thorax width (micropterous)
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: incomplete, with setae not closely and uniformly spaced
Fore wing second vein setal row: complete, setae uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fore wing first vein number of setae on distal half: 3
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Fore wing clavus - number of marginal setae: 5
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: dark
Fore wings: uniformly dark or shaded, but with base or sub-base pale or uniformly light brown
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Color of fore tarsi: pale or yellow, sometimes apical shaded or brown
Abdomen
Pleurotergites: not covered in microtrichia
Sternite II: with marginal setae and few discal setae
Number of discal setae on sternites III to VI: 14-20 (25)
Sternites IV, V and VI: with marginal setae and discal setae medially
Sternite VII median posteromarginal setae S1: arising in front of posterior margin
Sternite VII: with marginal setae and discal setae present on median area
Surface of lateral thirds of abdominal tergites: without regular rows of fine microtrichia
Number of lateral marginal setae on tergite II: 3
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Tergites IV and V median setal pair: shorter than distance between their bases
Tergites V to VII: with ctenidia laterally
Craspedum on tergites IV to VI: present, closely approximated, but independent, elongate lobes or teeth or with independent small, rounded triangular lobes
Craspedum on tergite VIII: without craspedum medially and toothlike microtrichia laterally
Tergite VIII ctenidia: posteromedial to spiracle
Tergite VIII posteromarginal comb of microtrichia: present and complete medially
Tergite VIII shape of posteromarginal microtrichia: long on broadly triangular bases
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
Microcephalothrips abdominalis is similar to species of Stenchaetothrips and Thrips, Fulmekiola serrata and Larothrips dentipes, because of the absence of ocellar setae I, the tergites V-VIII bear a pair of ctenidia laterally, which arranged on tergite VIII posteromesad to the spiracle. Compared to Microcephalothrips abdominalis, the species of Stenchaetothrips and Thrips have no posteromarginal craspedum on tergites, and the pronotum usually has 2 pairs of long posteroangular setae. Microcephalothrips abdominalis as well as Fulmekiola serrata and Larothrips dentipes, all of them exhibit a posteromarginal craspedum on tergites, but in Fulmekiola serrata and Microcephalothrips abdominalis the craspedum on tergites II-VII with of independent triangular lobes or teeth (Microcephalothrips abdominalis) or of medially rounded lobes fused at base and laterally large independent teeth (Fulmekiola serrata). Furthermore, in both the fore tibial apex is not extending around fore tarsus, both have the fore wing clavus with 4 (Fulmekiola serrata) or 5 marginal setae (Microcephalothrips abdominalis), and fore wing first vein with 3 discal setae. In contrast, in Larothrips dentipes the craspedum on tergites II-VII consist of unbroken border or flange, fore femora and fore tibiae are enlarged and fore tibiae with a stout curved claw ventrolaterally, the fore wing clavus possesses 6 marginal setae, and the fore wing first vein 2 discal setae. In contrast to Microcephalothrips abdominalis and Larothrips dentipes, Fulmekiola serrata has ocellar setae II much longer than III and longer than side of ocellar triangle, 2 pairs of elongate posteroangular setae and sternites without discal setae, but with craspeda on posterior margins. Microcephalothrips abdominalis and Larothrips dentipes with ocellar setae II about as long as ocellar setae III or shorter, no elongate (Larothrips dentipes) or 2 pairs (Microcephalothrips abdominalis) of moderately long posteroangular setae, and discal setae but no posteromarginal craspedum on sternites. Furthermore, Larothrips dentipes differs from Microcephalothrips abdominalis in having a tergite VIII posterior margin with a continuous craspedum medially but toothlike microtrichia laterally, sternites with a single row of 3-7 discal setae, and sternite VII median marginal setae arise at margin. But in Microcephalothrips abdominalis the posterior margin of tergite VIII present a complete comb of long microtrichia on broadly triangular bases, sternites with a double row of usually 14-25 discal setae, and sternite VII median marginal setae arise in front of margin.
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. The duration of the life cycle varies from 9-20 days (Ananthakrishnan 1971).
Host plants
Mainly Compositae (Asteraceae).
Crops: African spiderplant, amaranth, dahlia, French beans, marigold, potato, pot marigold, pyrethrum, sunflower, tomato.
Weeds: Achyranthes aspera, Ageratum conyzoides (tropical whiteweed), Bidens pilosa, Clitoria ternatea, Conyza bonariensis (Argentine fleabane), Flaveria australe, Euryops chrysanthemoides (African bush-daisy), Leonotis nepetifolia, Nycandra physalodes, Pentansia ouronogne, Pulicaria crispa, Senecio diversifolius, Tithonia diversifolia.
Vector capacity
None identified, but possible mechanical distribution of phytopathogenic fungi and bacteria.
Microcephalothrips abdominalis is reported to vector the pollen borne Tobacco Streak Virus (Greber et al. 1991, Sharman et al. 2010).
Damage and symptoms
The feeding activity of this thrips causes scars on the floral parts, the superficial layers getting completely destroyed and decolorized in severe infestation. Young buds are also infested and the developing florets shrivel up; such buds remain unopened (Ananthakrishnan 1971).
Detection and control strategies
Microcephalothrips abdominalis was more attracted to blue and white traps as compared to the yellow coloured traps (Chu et al. 2006). Varatharajan (1985) described a parasite-host interaction in relation to the nematode Anguillulina aptini (Sharga) - a parasite on Microcephalothrips abdominalis (Crawford) and Frankliniella schultzei (Trybom).
Additional notes
The sensoria on antennal segments III-IV are usually forked, although one half of these forks is sometimes difficult to observe. However, individuals from various localities have sensorium on one or both segments that are simple not forked, or more rarely with just the apex developed into a short fork. Variants of this sort have been described from China as separate species, but these are listed now as synonyms (Hoddle et al. 2008).
Microcephalothrips abdominalis pollinates plants like Tridax procumbens, Wedelia chinensis, Ageratum conyzoides and Cosmos bipinnatus (Ananthakrishnan et al. 1981; Gopinathan et al. 1981; Varatharajan et al. 1982).
Biogeography
Widespread throughout the tropics and subtropics. Egypt (Maadi),
Kenya, Nigeria (Ibadan),
South Africa (Mpumalanga: Groblersdal), Tanzania, Uganda.
African countries where Microcephalothrips abdominalis has been reported
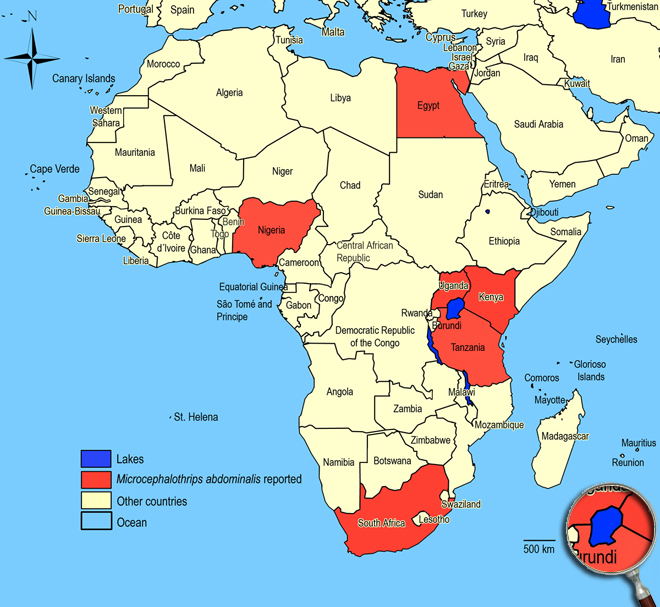
Occurence of Microcephalothrips abdominalis in East Africa
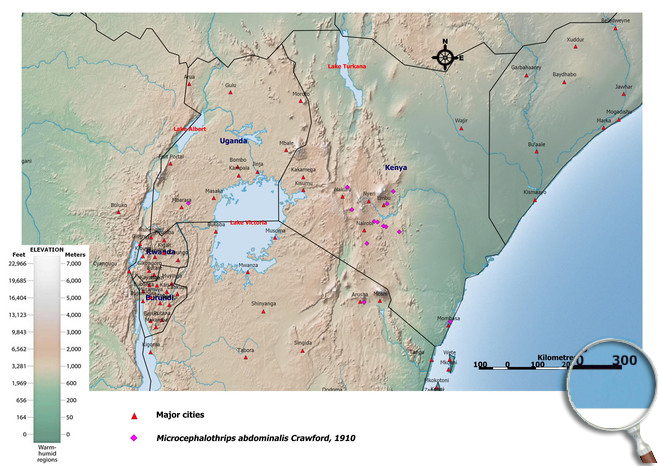
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Microcephalothrips abdominalis in parts of East Africa.

Bibliography
Ananthakrishnan TN (1956). Studies on some Indian Thysanoptera III. Zoologischer Anzeiger. 157: 130-139
Ananthakrishnan TN (1971). Thrips (Thysanoptera) in agriculture, horticulture & forestry - diagnosis, bionomics & control. Journal of Scientific and Industrial Research. 30 (3): 113-146
Ananthakrishnan TN, Varatharajan R & Gopinathan K (1971). Pollination in Wedelia chinensis (Osbeck) Merr and Tridax procumbens L. (Compositae) by Thrips (Thysanoptera: Insecta). Proceedings Indian national Science Academy B47 (2) 159-165
Chu CC, Ciomperlik MA, Chang NT, Richards M & Henneberry TJ (2006). Developing and evaluating traps for monitoring Scirtothrips dorsalis (Thysanoptera: Thripidae). Florida Entomologist 89: 47-54
Crawford DL (1910). Thysanoptera from Mexico and the South II. Pomona College Journal of Entomology. 2 (1): 153-170
Feng J, Nan X & Guo H (1998). Two new species of Microcephalothrips (Thysanoptera: Thripidae) from China. Entomotaxonomia. 20: 257-260
Feng J, Zhang J & Sha Z (2002). A new species of Microcephalothrips (Thysanoptera: Thripidae) from China. Entomotaxonomia. 24: 167-169
Girault AA (1927). Some new wild animals from Queensland. Published privately, Brisbane, 3 pp
Gopinathan K,
Varatharajan R &
Ananthakrishnan TN (1981). Incidence of Microcephalothrips abdominalis (Crawford)(Thys.,Insecta) in relation to the pollination biology of the weed Ageratum conyzoides L. (Compositae). Proceedings of Indian National Science Academy 47 47: 505-509
Greber RS, Klose MJ & Teakle DS (1991). High incidence of tobacco streak virus in tobacco and its transmission by Microcephalothrips abdominalis and pollen from Ageratum houstonianum. Plant Disease 75:450-452
Hoddle MS, Mound LA & Paris DL (2008). Thrips of California. Centre for Biological Information Technology, Queensland, Australia, CDROM
Jones PR (1912). Some new California and Georgia Thysanoptera. Technical series, USDA, Bureau of Entomology. 23 (1): 1-24
Karny H (1926). Studies on Indian Thysanoptera. Memoirs of the Department of Agriculture in India, Entomological Series. 9 (6): 187-239
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1926). New American Thysanoptera. Transactions of the American Entomological Society. 52 (2): 119-128
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp.
Mound LA & Marullo R (1996). The thrips of Central and South America: An introduction (Insecta: Thysanoptera). Memoirs on Entomology, International, Vol. 6. Associated Publishers, Gainsville, 487 pp
Palmer JM (1990). Identification of the common thrips of Tropical Africa (Thysanoptera, Insecta). Tropical Pest Management. 36 (1): 27-49
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Pitkin BR & Mound LA (1973). A catalogue of West African Thysanoptera. Bulletin de ľInstitut Fondamental ďAfrique Noire, Série A. 35 (2): 407-449
Priesner H (1923). A. Dampfs Aegypten-Ausbeute: Thysanoptera. Entomologische Mitteilungen. 12 (2): 115-121
Priesner H (1938). Contributions towards a knowledge of the Thysanoptera of Egypt, XI. Bulletin de la Société Royale Entomologique ďEgypte. 21: 208-222
Raizada U (1969). Some observations on the bionomics of Microcephalothrips abdominalis (Crawford) (Thysanopt., Thripidae). Indian Journal of Entomology. 31: 336-341
Sharman M, Persley DM & Thomas JE (2010). Epidemiology of Tobacco streak virus in Queensland, Australia. In: Proceedings of the 1st Australian Summer Grains Conference, 21st-24th June 2010, Gold Coast, Australia.
Stannard LJ (1968). The thrips, or Thysanoptera, of Illinois. Illinois Natural History Survey Bulletin. 29 (4): 214-552
Varatharajan R (1985). Parasite-host interaction in relation to the nematode Anguillulina aptini (Sharga) - a parasite on Microcephalothrips abdominalis (Crawford) and Frankliniella schultzei (Trybom). Current Science 54(8): 396-398
Varatharajan R, Gopinathan K & Ananthakrishnan TN (1982). Comparative efficiency of thrips in relation to other foraging insects in the pollination of Cosmos bipinnatus Cav. (Compositae). Proceedings of Indian national Science Academy B48 (6): 735-739
Vierbergen G, Cean M, Szeller IH, Jenser G, Masten T & Simila M (2006). Spread of two thrips pests in Europe: Echinothrips americanus and Microcephalothrips abdominalis (Thysanoptera: Thripidae). Acta Phytopathologica et Entomologica Hungarica. 41: 287-296
Watson JR (1922). On a collection of Thysanoptera from Rabun County, Georgia. Florida Entomologist. 6 (3): 34-39
Watson JR (1931). A collection of Thysanoptera from western Oklahoma. Publications of the University of Oklahoma. 3 (4): 339-345
zur Strassen R (1983). Thysanopterologische Notizen (6) (Insecta: Thysanoptera). Senckenbergiana Biologica. 63 (3-4): 191-209
zur Strassen R (2003). Die terebranten Thysanopteren Europas und des Mittelmeer-Gebietes. Die Tierwelt Deutschlands und der angrenzenden Meeresteile nach ihren Merkmalen und nach ihrer Lebensweise, 74. Teil. Goecke & Evers, Keltern, Germany, 277 pp
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California